This week is dedicated to the safety of medicines through the #MedSafetyWeek 2024 campaign. The perfect opportunity to review some essential aspects of the Claude Bernard drugs database, the unique tool that helps and secures the prescription and dispensing of medicines.
Beyond the main elements that recommend the Claude Bernard drugs database – attributes, functionalities, advantages – we are happy to introduce you to some lesser-known aspects about it, such as the story of the name Claude Bernard, the evolution of the database over time and the work of the team that is behind the ambitious Claude Bernard project in Romania.
The main reasons to consult the Claude Bernard drugs database
Claude Bernard is a complete and safe drugs database, implicitly integrated in the software applications that Cegedim RX develops for doctors (Pharmec Cabinet, Pharmec Nova), and pharmacists (Pharmec Farmacie).
Through it, healthcare professionals in Romania benefit from:
- An electronic medical assistant that audits drug prescribing and dispensing, providing accurate and up-to-date information on full product monographs, dosage and therapeutic equivalences;
- The only drugs database that displays interactions related to the patient’s medical condition (related to age, known allergies, pregnant/breastfeeding etc.) by diagnosis code.
By consulting the Claude Bernard database, doctors and pharmacists have immediate access to both reliable, centralized and daily updated medical information, as well as real-time alerts based on patient profile and drugs interactions. All for safer prescribing and dispensing, but also allowing healthcare professionals to save time and provide the best care to their patients.
Brief history of the Claude Bernard drugs database
Launched in France in 1985, the Claude Bernard database is the first computerized drugs database. It was launched by the French company RESIP from a research prototype on a structured drug database developed at the Claude Bernard Faculty of Medicine in Lyon.
Combining technological and scientific expertise for healthcare, the Claude Bernard drugs database was named after the famous French physician and pharmacist, known as the founding father of modern physiology and experimental medicine.
In 2001, RESIP and the Claude Bernard database became part of the Cegedim group, and in 2018 the Claude Bernard drugs database was also launched in Romania. Over the years, it obtains multiple certifications and becomes an internationally recognized solution, offering the guarantee of accurate, scientifically validated information to support medical decision making.

Launch of the Claude Bernard database in Romania, National Pharmacy Congress – CNFR 2018
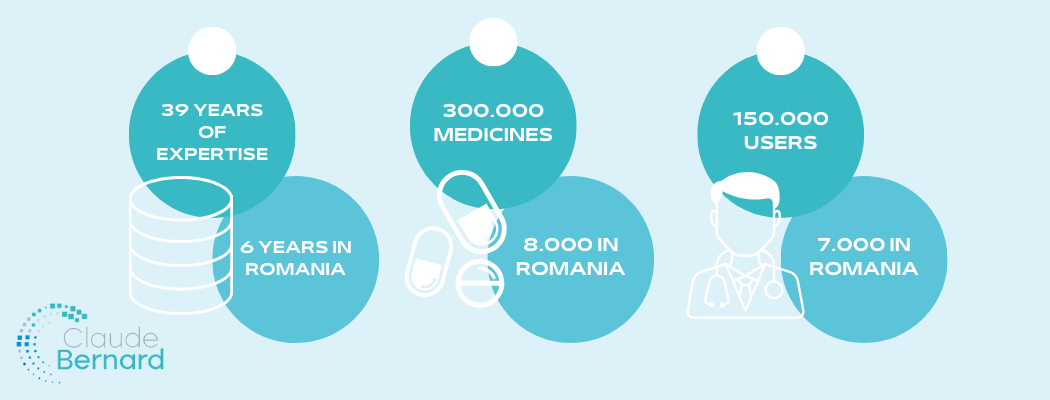
A few key figures for the Claude Bernard drugs database
To complete the overview of this tool of real help to doctors and pharmacists, here are some key figures that define the Claude Bernard base:
- Almost 40 years of medical and technical expertise, 6 years since its official launch in Romania
- 300.000 medicines and health products referenced and updated daily, with almost 8.000 active positions for products marketed in Romania
- 150.000 healthcare professionals around the world, out of which around 7.000 in Romania, who trust it every day in their practice
The team behind the Claude Bernard drugs database in Romania
Based on the experience of our French colleagues for several decades now, a team of healthcare professionals and specialists from Cegedim Healthcare Romania has been, during the past few years, exclusively dedicated to the adaptation and updating of the Claude Bernard drugs database for Romanian doctors and pharmacists.
Led by Dr. Ana Comănescu, the team of ten doctors and pharmacists constantly ensures:
- Data accuracy: all RX and OTC drugs in the Claude Bernard database are constantly updated with official information from the summary of product characteristics (SPC)
- Regulatory compliance: the possible alert messages issued by the Claude Bernard drugs database are updated in accordance with the legislation in force and the products’ dosage in Romania
Although the official Medex (CaNaMed) list of CIM codes (W) contains several tens of thousands of positions, some of them inactive, the Claude Bernard drugs database currently contains around 7800 active positions (out of which around 1500 are OTCs), for products that have been sold on the Romanian territory.
The constant efforts to maintain and update all this information, in accordance with the official data from the National Agency of Medicines and Medical Devices in Romania (ANMDMR), make the Claude Bernard database a reliable tool for healthcare professionals in Romania.
